
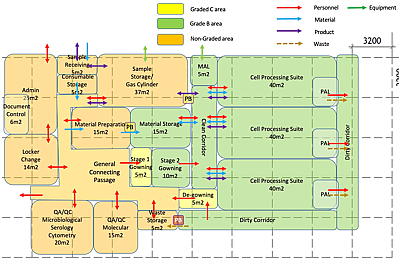
Architectural block diagram: laboratory space programming
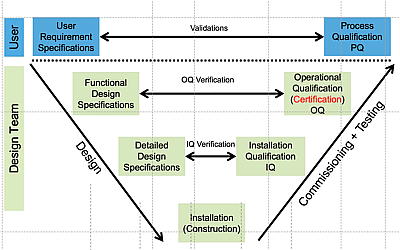
V-Diagram: laboratory design and quality control
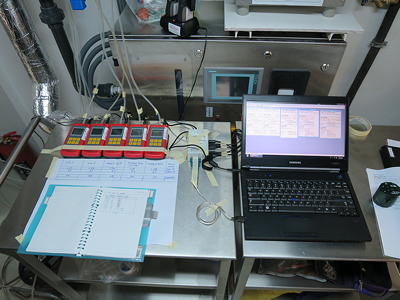
Certification of inward airflow (pressure cascade, BSL3 lab)
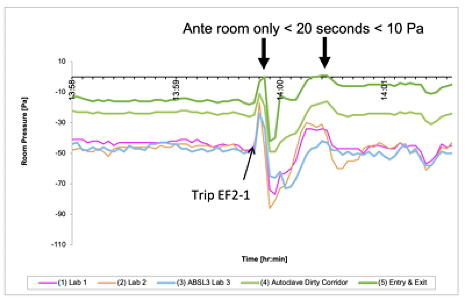
Differential pressure trending (BSL3 lab)

Full protection suit in a BSL-3Ag lab

BSL2 and BSL3 hands-on training

Ferret-Isolator (BSL3+)
|
[Deutsch]
Basler & Hofmann Switzerland team suppports you as global biosafety consultants.
For any enquiries you may have, please reach out to Felix Gmünder at:
Services and competences
We find and/or develop solutions for your biosafety laboratory (BSL-2) and high containment laboratory project (BSL-3, BSL-4). We specialise in combined GMP / BSL-2 or BSL-3 facilities, in particular polio vaccine production facilities conforming to WHO GAP IV.
Our services include but are not limited to:
- Conceptual design and planning
- Risk assessments e.g., according to WHO 2020
- Selection of primary containment equipment and laboratory outfitting
- Review and assistance of schematic design
- Technical due diligence, gap analysis, renovation, retrofitting
- Support maintenance incusive maintenance concepts
- Review of tenders, support construction supervision
- Support testing & commissioning, handover
- Certification and validation
- Biosafety training and hands-on training in the laboratory
- Development and setting up and implemenation biorisk managment systems and programs, e.g., WHO 2020 and ISO 35001
- SOP and work instruction development
- Comprehenisve laboratory safety (including OHS, ergonomics)
- Safety audits
Credentials and experience of our lead consultant Felix Gmünder include:
- 20+ years experience in BSL-3 and BSL-4 laboratory design and consulting
- Member of the Swiss Expert Committee for Biosafety
- Singapore Ministry of Health Approved BSL-3 Certifier
- Ph.D., M.Sc. Swiss Federal Institute of Technology (Microbiology)
- Registered Biosafety Professional (RBP, ABSA)
- Certified Safety Engineer (OHS)
Reference projects
Reading materials & recommendations
- Pressure Testing for BSL-3 and BSL-4 Laboratories
This article of the Containment Talk series addresses the requirement for pressure testing in BSL-3 and BSL-4 laboratories. Containment boundary walls, partition walls, ceilings, and penetration systems must withstand pressure surges that may occur during HVAC testing and maintenance activities, and HVAC equipment and power failures. These surges pose a significant risk: Documented cases have shown that they can lead to severe structural damage, including the bursting or collapse of facilities. In addition, containment boundaries and partition walls must ensure long-term leaktightness, maintaining suitability for fumigation despite the effects of aging and mechanical stress.
- Design of ABSL-3 and Other Special Animal Facilities: Logistics Studies Using Spaghetti Diagrams
This Containment Talk article presents the application of Lean Six Sigma and the Spaghetti Diagram methodology to optimise the design and logistics of laboratory and veterinary animal testing facilities in general, and high- and maximum-containment facilities in particular. These tools ad-dress design challenges in the areas of biosafety, occupational health and safety, animal welfare, hygiene standards, workflow efficiency, and compliance with regulations and best practices.
- Special Laboratory Design and Construction Models with a Focus on Animal and Biosafety Laboratories
Designing an animal, biosafety or other special laboratory isn't just about architecture and engineering—it's about sustainable safety and functionality. Yet in many countries, especially those with limited resources, there is no formal model, framework or process for the planning, design and construction for these critical facilities. Containment Talk article 14 summarises the most important and critical elements of the plan of works for animal, biosafety, and other special laboratories.
- Laboratory Decommissioning in a Nutshell
This Containment Talk gives an overview of the main steps required for the decommissioning of laboratories. Due to the case-specific differences, the decommissioning of laboratories is always project-specific and risk-based. The approach presented provides a general guideline for the development of a decommissioning strategy and a decommissioning plan, as well as for their implementation.
- Contract Models and Delivery Methods for BSL-3 and BSL-4 Laboratories
Contracting for planning, design, and construction of BSL-3 and BSL-4 facilities requires a thoughtful approach if the outcome is to match the intentions of the owner or the principal, and the users. From a project risk perspective, selecting the appropriate contract model and delivery method is crucial. It should be noted that none of the biosafety guidelines address contract models and delivery methods. The choice of approach must be made before the project design phase begins. This Containment Talk discusses the three contract models and delivery methods suitable for the design and construction of BSL-3 and BSL-4 biocontainment facilities, along with tips and advice on how to get the most out of them.
- BSL-3 Laboratory Maintenance With SOP Templates
It is essential that all laboratories have a maintenance strategy or plan in place. Given the intricate nature of BSL-3 laboratories, it is imperative to dedicate particular focus to maintenance. The objective of the maintenance plan is to
_ guarantee the safety of people and the environment at all times,
_ prevent serious malfunctions and failures, and
_ maintain the value of the facility in a long-term and sustainable manner. This Containment Talk article presents an example of a cutting-edge maintenance plan and SOP templates that meets the requirements of ISO 35001 "Biorisk management for laboratories and other related organisations", the WHO monograph "Laboratory design and maintenance" and other pertinent standards and guidelines.
- BSL-3 and BSL-4 Laboratory Fumigation Strategies
BSL-3 and BSL-4 facilities must be designed and constructed in such a way that the containment and the contaminated parts of the ventilation system, including the exhaust HEPA-filter stations and enclosed systems (biosafety cabinets Class III, ducts, etc.) can be safely decontaminated by fumigation. In this Containment Talk article, possible fumigation strategies and methods are discussed with their advantages and disadvantages.
- Fire Protection and Safety in BSL-3 and BSL-4 Laboratories
When designing BSL-3 and BSL-4 laboratories, fire detection, suppression and fighting will be part of every project. This Containment Talk article is an introduction for stakeholders and presents the basics for finding a solution that is effective, compliant with local fire codes, and affordable.
- BSL-4 Laboratory Layers of Protection
In this Containment Talk article on BSL-4 laboratories, several layers of protection are discussed. Unlike Biosafety Level 2 and 3 laboratories, it is theoretically possible in BSL-4 facilities to completely isolate the hazard from laboratory personnel and the environment through structural and engineering risk controls. If a BSL-4 facility is designed, constructed, operated and maintained according to the state of the art, the human factor and errors become less dominant.
- Understanding ‘Airtight’ and ‘Gastight’ in Relation to the Design and Construction of BSL-3 and BSL-4 Facilities
This Containment Talk takes you on a journey into the wilds of airtight, gastight and similar, poorly defined terms used in every BSL-3 and BSL-4 design, construction, and retrofit project. What does airtight, gastight mean for doors, wall-integrated equipment or penetrations? A simple and practical solution to this problem is presented.
- BSL-2 and BSL-3 Laboratory Air Exchange Rates: A Novel Proposal
This Containment Talk introduces laboratory dilution ventilation systems and the concept of air exchange rate (ACH) in general and presents a proposal to reduce ACH in BSL-2 and BSL-3 microbiological and biomedical laboratories without compromising safety in routine and non-routine operations (spill management) by considering the filtering effect of Class IIA2 recirculating biosafety cabinets. For laboratory concept design, a ventilation design flowchart is also included.
- Autoclave Validation for Waste Decontamination in BSL-2, BSL-3, and BSL-4 Laboratories
Autoclave validation in microbiological and biomedical laboratories at biosafety levels 2, 3, and 4 is a requirement-but there are no current guidelines or regulations on how to validate and monitor the steam decontamination process. The focus of this article is on the decontamination of liquid and solid biohazardous waste for safe disposal into the municipal waste stream (local regulations may apply).
- Biosafety Cabinets - Making the Most of It: Keeping Products Protected and Sterile
This article explains how cross-contamination in the biosafety cabinet can occur and how it can be minimised. The information is relevant to laboratory personnel who are concerned with biosafety, protecting products from contamination, maintaining purity and sterility, or all of the above. Aseptic work conditions are required, e.g., for cell and tissue culture, sterile specimen handling in clinical laboratories and the maintenance of microbiological stock cultures.
- BSL-3 Laboratory Certification - An Introduction and Overview.
BSL-3 certification or verification is an important post-construction element prior to handover to the owner and operator. It is carried out by an independent third party certifier. This article describes the purpose, procedure as well as methods and scope.
- The Swiss Cheese Risk Assessment Model for BSL-2 Laboratories
This article looks at the multiple layers of protection or defence-in-depth concept to protect BSL-2 laboratory workers and the environment from biohazards.
- Risk Management for BSL-3 Laboratories Through Multiple Layers of Protection
This article looks at the multiple layers of protection or defence-in-depth concept to protect BSL-3 laboratory workers and the environment from biohazards.
- In English, en français, in Italiano: Recommendation on Structural and Technical Safety Measures in BSL-3 Laboratories. This recommendation was prepared by Basler & Hofmann.
- Recommendation of the SECB on the Maintenance of BSL-2 and BSL-3 Laboratories.This recommendation was developed by Basler & Hofmann.
- BIBO HEPA-Filters in BSL-3 Labs: Yes or No?
- State-of-the-art safety engineering for BSL-3 labs (pptx, presented to the 3rd International Conference on Biosafety and Laboratory)
- Think outside the biosafety box (pptx, requirements for BSL2 facilities from the safety and biosafety perspectives; presented to the Swiss Biosafety Network (SBNet) annual conference)
- Design a Functional Laboratory
- BSL-3 Laboratory Engineering Consulting: Good Practice BSL-3 Engineering Design
- Certification Standards: BSL-2 and BSL-3 Laboratories
- BSL-3 Certification Requirements: Basler & Hofmann Inspection Scope
Videos
|







